June 1, 2020
IMPORTANT SAFETY INFORMATION
Impax Laboratories, LLC
Epinephrine Injection, USP Auto-Injector 0.3 mg
PRODUCT SAFETY ADVISORY
Epinephrine Injection, USP Auto-Injector 0.3 mg may be missing its
yellow “stop collar,” potentially causing medication overdose.
Dear Consumer:
Impax Laboratories, LLC, a wholly owned subsidiary of Amneal Pharmaceuticals LLC, is providing important safety information concerning its Epinephrine Injection, USP Auto-Injector 0.3 mg. Impax is writing to inform you that some Epinephrine Injection, USP Auto-Injector 0.3 mg devices may not contain the yellow “stop collar” component (see pictures below). The yellow “stop collar” is one of several components that work together to assure proper dosing of the auto-injector. If the auto-injector is missing a yellow “stop collar” component, it has the potential safety risk of delivering a double dose of the product to a consumer. An overdose of epinephrine has the potential to cause severe patient harm or death.
If you have received Amneal or Impax’s Epinephrine Injection, USP Auto-Injector 0.3 mg after December 20, 2018, Impax is requesting that you immediately perform a visual inspection (Steps 1-5) as described below to confirm the presence of the yellow “stop collar”:
- Carefully inspect for the presence of the yellow “stop collar.”
- If yellow “stop collar” is present, then the product is safe to use. No further action is necessary.
- If the yellow “stop collar” is missing, call or e-mail Amneal Drug Safety Department using the contact information below, for instructions for the return and replacement of the auto-injector:
AMNEAL PHARMACEUTICALS DRUG SAFETY DEPARTMENT
Phone: 1(877) 835-5472
Email: Drugsafety@amneal.com
Mail: Amneal Pharmaceuticals, 50 Horseblock Road, Brookhaven, New York 11719
Note: If you have any questions about the steps below, have difficulty following the instructions, or are unsure if the yellow “stop collar” is missing, please call AMNEAL PHARMACEUTICALS DRUG SAFETY DEPARTMENT
VISUAL INSPECTION INSTRUCTIONS TO CONFIRM PRESENCE OF YELLOW “STOP COLLAR”
Step 1: Remove the auto-injector from the carrying case.

Step 2: Place the auto-injector on a flat surface as shown below.

Step 3: Locate the edge of the label that states “Peel here for further instructions”. Lift the label edge until you see the clear part of the auto-injector.

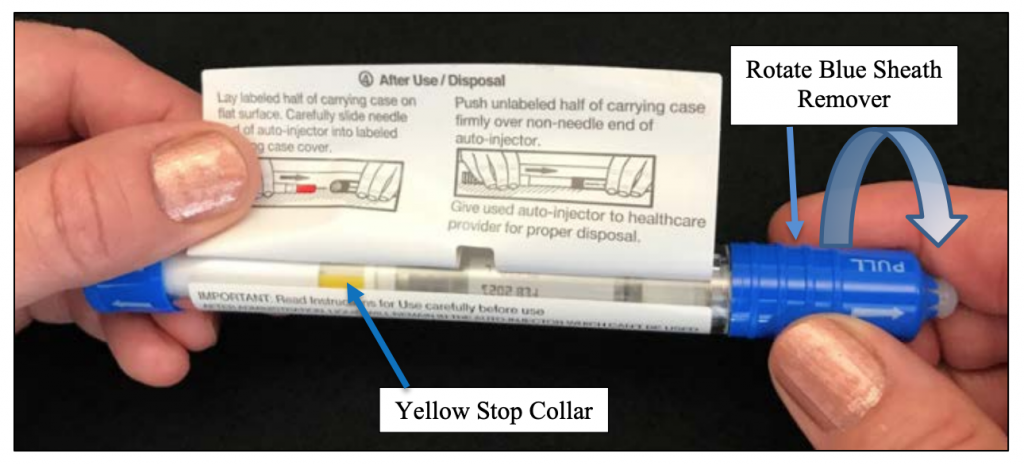
Step 4: Look for the YELLOW “stop collar”. See arrow below.

PLEASE NOTE: If the yellow “stop collar” is missing, hold the unit (as shown below) and gently rotate the blue sheath remover and observe until the yellow “stop collar” comes into view. DO NOT PULL OR REMOVE THE BLUE SHEATH REMOVER.
IMPORTANT:
- If yellow “stop collar” is present, then the product is safe to use. No further action is necessary.
- If the yellow “stop collar” is missing, contact Amneal Drug Safety Department using the contact information below, to make arrangements for the return of the auto-injector, and a replacement at no additional cost:
AMNEAL PHARMACEUTICALS DRUG SAFETY DEPARTMENT
Phone: 1(877) 835-5472
Email: Drugsafety@amneal.com
Mail: Amneal Pharmaceuticals, 50 Horseblock Road, Brookhaven, New York 11719
Step 5: Re-wrap the label to its original position and place the auto-injector into the carrying case.

To report issues with product, contact Amneal at:
AMNEAL PHARMACEUTICALS DRUG SAFETY DEPARTMENT
Phone: 1(877) 835-5472
Email: Drugsafety@amneal.com
Mail: Amneal Pharmaceuticals, 50 Horseblock Road, Brookhaven, New York 11719
Please note that adverse events or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or fax: download form www.fda.gov/MedWatch/getforms.htm or call
1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Thank you in advance for your cooperation.

Candis Edwards
SVP, Regulatory Affairs
Amneal Pharmaceuticals
50 Horseblock Road
Brookhaven, New York 11788
Office: 631.952.0214 X 338
Email: cedwards@amneal.com
#SnackSafelyAtHome





